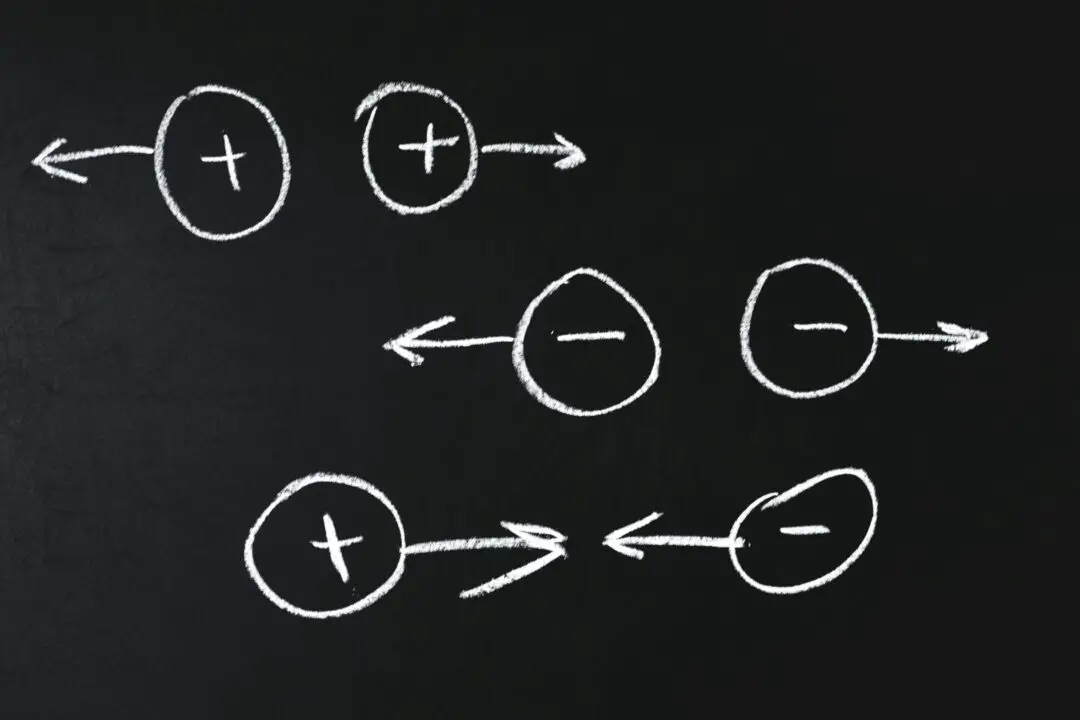
This article is about charging by friction or rubbing. We already know that electrical charges are a fundamental or natural property of a fundamental particle and that there are two types of charges, namely negative and positive charges. There are three ways in which electrons are transferred to charge the object, and they are
- friction,
- conduction, and
- induction.
What is charging by friction?
Rubbing, as the term suggests, is pushing two objects back and forth toward each other. The best way to feel the electrical charge is to rub those bodies together. Rubbing or friction allows electrons to travel. This gives a positive charge to one substance and a negative charge to another. Charges remain on the surfaces of the products until they can flow or discharge. Whenever there is friction between materials that differ in their ability to give up or gain electrons, the electrons can be transferred in this manner, i.e. through friction.

Charging by friction Explanation
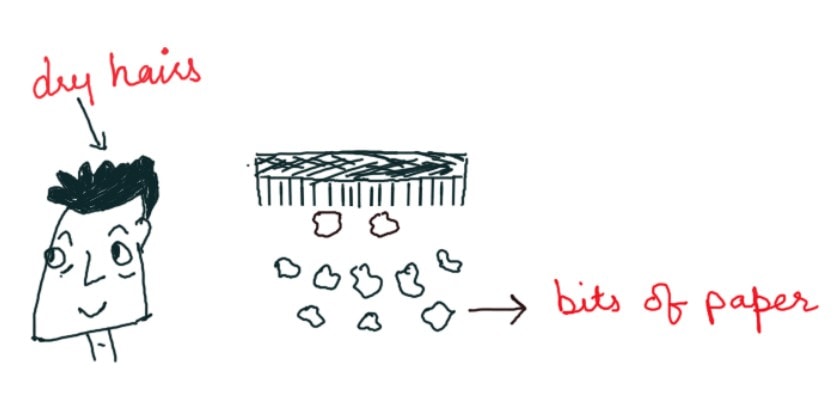
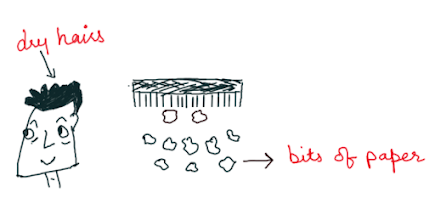
If we run a comb through our hair, the comb gets charged and can attract tiny pieces of paper. This is because the comb may have lost its electrons or gained some electrons when we rub it with the scalp. This comb is now a charged body. The net charge on the comb interacts with the net charge on small pieces of paper resulting in attraction. Many such solid materials are known to attract light objects like light feathers, pieces of paper, straw, etc.
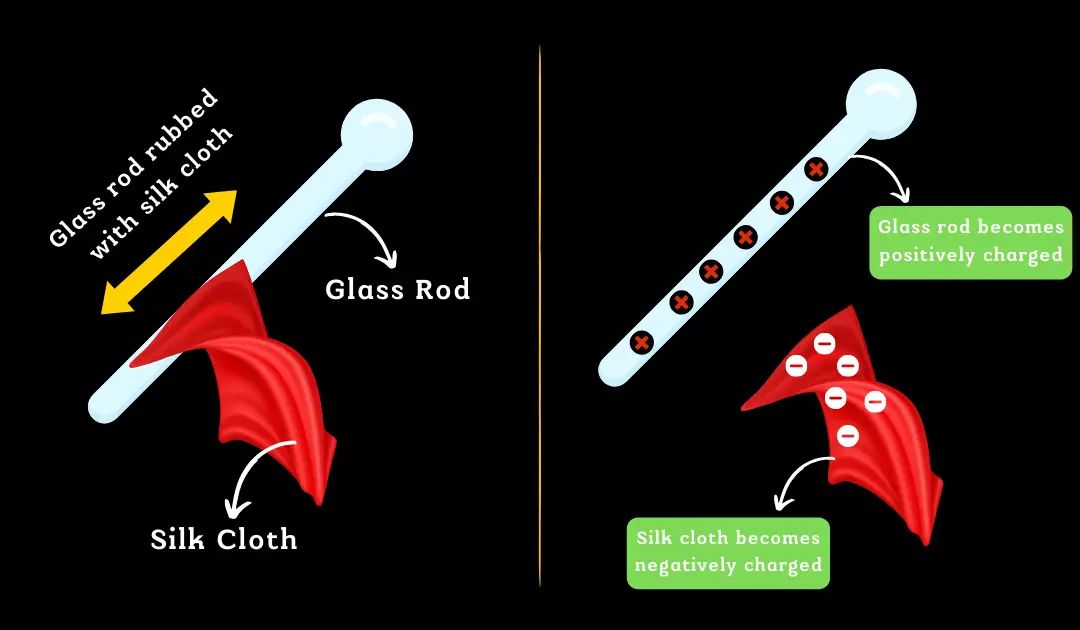
Take another example of a glass rod and a silk fabric. Now if we rub a glass rod with a silk cloth, the silk cloth absorbs electrons from the glass rod and gets negatively charged. As a consequence, the glass rod is positively charged due to the loss of electrons.
Similarly, there are many other materials that are electrically charged when they are rubbed against each other for example, when we rub the woolen cloth with ebonite or very hard rubber rod, the woolen cloth becomes positive and ebonite rod becomes negatively charged.
The interpretation of the presence of the electrical charge on the rubbing is clear. Material bodies consist of many electrons and protons in similar numbers and are thus neutral in their natural state. But when a glass rod is rubbed with a silk cloth, electrons are shifted from a glass rod to silk cloth. The glass rod is positively charged and the silk fabric is negatively charged as it absorbs additional electrons from the glass rod. In this case, bar after rubbing, comb after running through dry hair becomes electrified and this is an example of frictional electricity.
It is important to remember here that only non-metallic bodies can be charged by friction. The explanation is that the charges are free to travel within the material in a metal or conductor. If you apply a charge to one component of the conductive material, the other charges will be automatically re-arranged to neutralize the charge.
In the case of non-metallic or non-conductors, the charges you put on the surface of the material by rubbing remain as there is no re-arrangement taking place. This is because the insulator charges do not pass freely inside the content.
charging by friction examples
Charging by friction, also known as triboelectric charging, occurs when two materials come into contact and electrons are transferred from one material to another, resulting in one material becoming positively charged and the other negatively charged. Here are some examples of charging by friction that might be observed at home or in daily life:
- Rubbing a balloon on your hair: When you rub a balloon on your hair, electrons are transferred from your hair to the balloon, making the balloon negatively charged and your hair positively charged.
- Shuffling your feet on a carpet: When you shuffle your feet on a carpet, electrons are transferred from the carpet to your shoes, making your shoes negatively charged and the carpet positively charged. This can result in a static shock when you touch a grounded object.
- Removing clothes from the dryer: When clothes tumble together in the dryer, electrons are transferred between the different fabrics, resulting in static cling and sometimes small electric shocks when you touch the clothes.
- Rubbing a glass rod with a silk cloth: When you rub a glass rod with a silk cloth, electrons are transferred from the glass to the silk, making the silk negatively charged and the glass positively charged.
- Rubbing a plastic comb through your hair: When you comb your hair with a plastic comb, electrons are transferred from your hair to the comb, making the comb negatively charged and your hair positively charged.
- Rolling a rubber ball on a woolen surface: When a rubber ball rolls on a woolen surface, electrons are transferred from the wool to the rubber, making the rubber ball negatively charged and the wool positively charged.
- Rubbing an amber rod with fur: When you rub an amber rod with fur, electrons are transferred from the fur to the amber, making the amber negatively charged and the fur positively charged. This is a classic example often used to demonstrate the phenomenon of charging by friction.
Related Articles