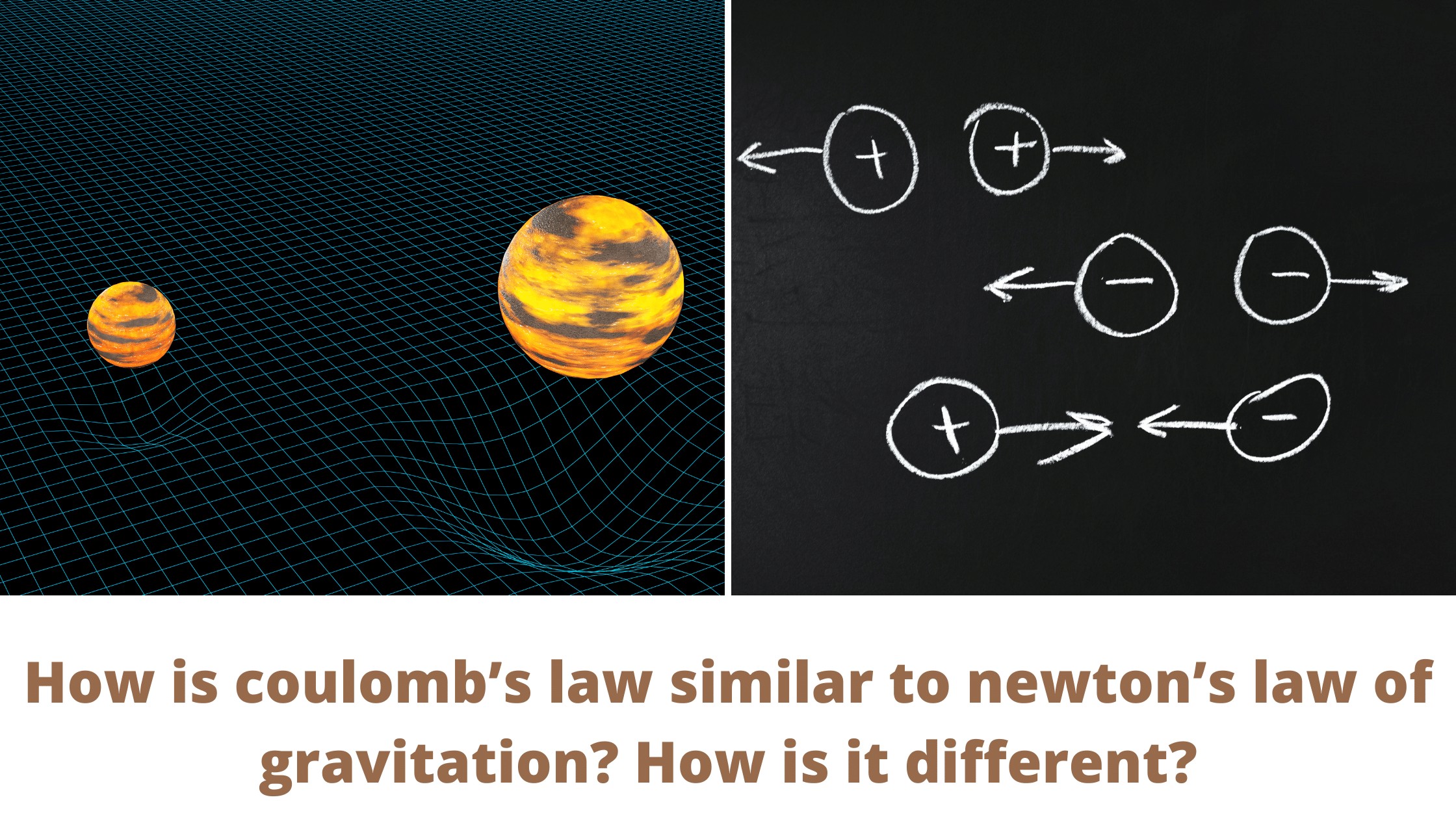
If you have learned about both Coulomb’s law of electrostatics and Newton’s law of gravitation while studying mechanics you might be making a comparison about both of them as their formula looks somewhat similar in their form.
In this article, we will look at the similarities and differences between these two laws. We would look at how is coulomb’s law similar to newton’s law of gravitation? How is it different?
Let us first have a look at both these laws.
What is Coulomb’s Law?
Coulomb’s law in electrostatics explains how static electric charges interact with each other when they are in each other’s field of influence. This law states that
The force of attraction or repulsion between two stationary electric point charges is directly proportional to the product of the magnitude of two charges and is inversely proportional to the square of the distance between them. This force acts along the line joining the two charges.
Check your knowledge about coulomb’s law through this Coulomb’s law quiz
Consider two electric charges $q_1$ and $q_2$ which are separated by a distance $r$ then according to this law,
$F \propto {q_1}{q_2}$ and $F \propto \frac{1}{r^2}$
Therefore,
$F \propto \frac{q_{1}q_{2}}{r^{2}}$
or,
$F = k\frac{{{q_1}{q_2}}}{{{r^2}}}$
where, $k$ is the constant of proportionality. The value of $k=8.85 \times 10^{-12}C^2/Nm^2$ in vacuum.
What is Newton’s law of gravitation?
This law explains the interaction between two massive bodies.
This law states that each and every massive body attracts every other body in the universe with a force that is directly proportional to the product of their masses and inversely proportional to the square of the distance between them.
Mathematically
$F\propto \frac{m_{1}m_{2}}{r^2}$
Newton’s principle of gravitation can be formulated as follows:
$\Rightarrow F=G\frac{m_{1}m_{2}}{r^2}$
where $m_1$ and $m_2$ are the masses of two bodies separated by a distance $r$ and G is the universal gravitational constant. Value of $G= 6.673 \times 10^{-11} N m^2/kg^2$.
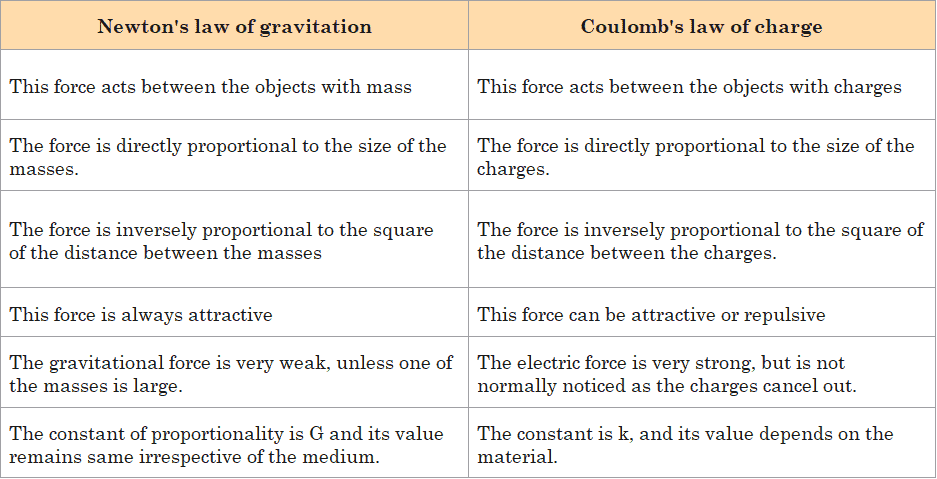
Similarities between Coulomb’s Law and Newton’s law for gravity
We find the following similarities when we compare both these laws
- The charge ‘q’ works the same way in Coulomb’s law as the mass ‘m’ does in Newton’s law of gravitation.
- Forces are described by both of these laws. The force between electric charges is defined by Coulomb’s law, while the force between masses is defined by Newton’s law of gravitation.
- They are both inverse square laws. This means that the In case of electric forces is inversely proportional to the square of the distances between charges. Similarly, in the case of Newton’s law of gravitation, there is the same inverse proportionality between the force and the distance of masses.
- Both laws describe central forces, that is, forces that operate along the line connecting the two charges in the case of Coulomb’s law and the two masses in the case of Newton’s law of gravitation.
- The forces identified by both laws are conservative forces, which means that the work done on any object by these forces is independent of the direction taken by the objects. It is determined by the initial and final position of the object under consideration only.
Differences between them
- The electrostatic force is medium-dependent and can be shielded, whereas the gravitational force does not have this property.
- Since the sign of mass is always positive, the gravitational force is always attractive, while the electric force can be either attractive or repulsive because the sign of electric charge can be either positive or negative.
- Here it is important to note that, a positive force denotes a repulsive force, while a negative force denotes an attractive force. Gravitational forces are always attractive in nature. Coulomb’s Law specifies the sign of the force depending on the signs of the two charges. Similar charges will produce a positive force that would be repulsive in nature. Opposite charges will return a negative force that is attractive in nature.
- Since the gravitational constant G has a much lower value than the Coulomb’s constant, the gravitational force is much weaker than the electric force.
Comparison of newton’s law of gravitation and coulomb’s law
Given below is the comparison between these two laws in tabular form
Test yourself with this quiz