In this article learn about What do electric forces between charges depend on. We will explain the concept in detail. Read on to get your answer.
What do electric forces between charges depend on?
Electric forces between two charges depend on
1. the distance between the charges
2. amount of charge they both have
3. medium in which they are kept
4. Nature of the charges
Elementary particles form the matter surrounding us. The three most common elementary particles are electrons, protons, and neutrons.
These elementary particles possess distinctive physical properties like mass, electrical charge, and spin. Due to these physical properties, elementary particles exhibit physical phenomena like gravitational pull and electrical forces.
For example,
- Due to the mass of elementary particles, they exert a gravitational pull on each other.
- Thus, an electron with me= 9.10940 x 10-31 kg attracts another electron placed 1 cm away with a gravitational force of 5.5 x 10-67 N.
But, do electrons really attract each other? No, they don’t. Instead, they repel each other. This repulsive force is due to the charge possessed by the elementary particles.
What is an electrical charge?
Electrical charge is the fundamental physical property of a matter that causes it to experience a force when placed in an electromotive field.
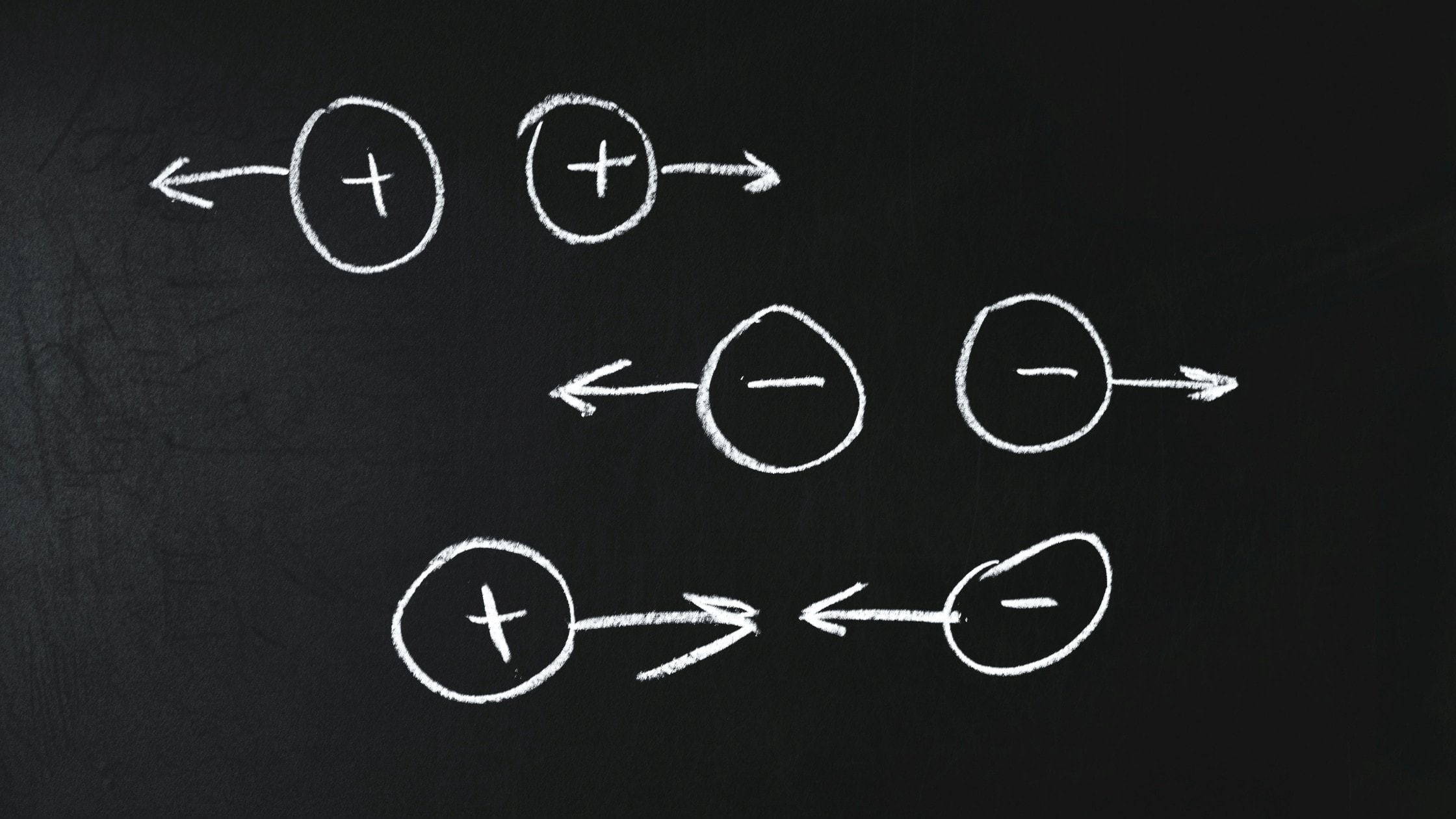
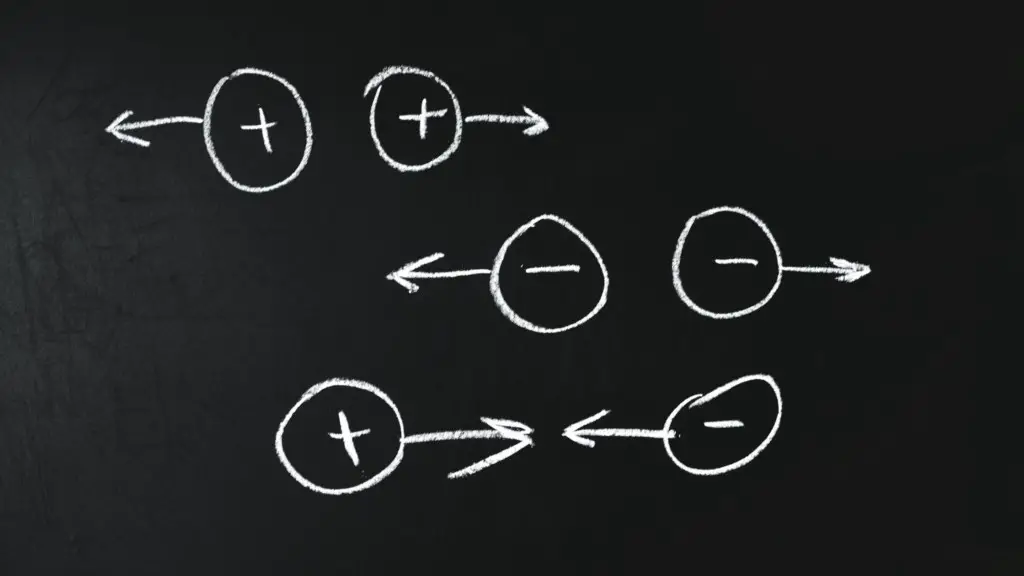
A charge can be of two kinds; positive and negative.
A positive charge signifies that the electrical force exerted is attractive, While a negative charge exerts a repulsive force.
SI unit of charge is coulomb abbreviated as $C$.
A charge on a proton is considered to be positive, while that on an electron is taken as negative. A neutron is an uncharged elementary particle.
Why do two electrons repel each other?
Though two electrons separated by a 1 cm distance exerts a gravitational force of 5.5 x 10-67 N due to their mass. Yet, due to the negative electrical charge between the two electrons, they exert a repulsive force of greater magnitude on each other.
Therefore, two electrons placed at a 1 cm distance in an electrical field repel each other with a 2.3 x 10-24 N force.
What factors determine the electrical forces between the charges?
French physicist Charles-Augustin de Coulomb in 1915 described the factors influencing the electrostatic force between the charged particles in the form of Coulomb’s law.
Coulomb’s law quantifies the magnitude of the force between the charged particles.
It states that “the magnitude of the electrostatic force between two electrically charged particles is directly proportional to the product of the magnitude charges and inversely proportional to the square of the distance between them.
Mathematical it is expressed as,
$F \propto \frac{q_{1}q_{2}}{r^{2}}$
Therefore,
$F = k\frac{{{q_1}{q_2}}}{{{r^2}}}$
Where,
- q1 and q2 are the charges on the particles.
- r is the separation between them
- k is a proportionality constant.
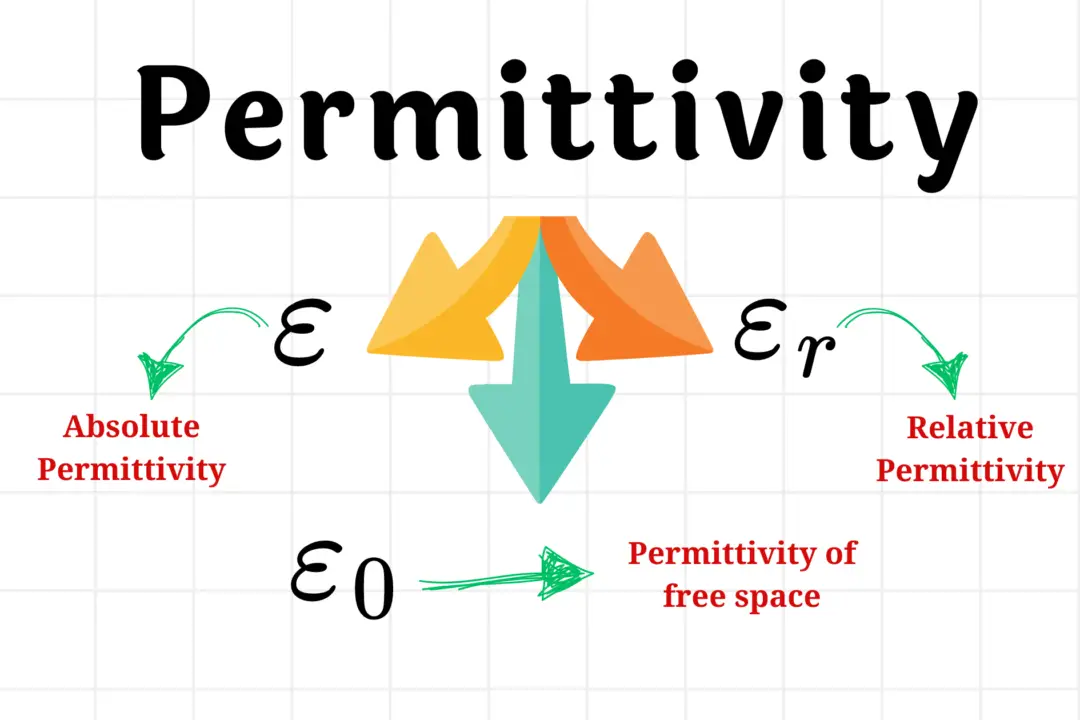
- The value of k is equal to 1/4πε0. The constant ε0 is called the permittivity of free space and its value is 8.85419 x 10-12 C2 N-1 m-2 in SI units.
- Therefore the value of k in SI units is defined as 8.98755 x 109 N m2 C-2.
- The value of k is constant for a particular medium.
Thus, the factors affecting the electrical force between charges can be summarized as :
- (i) Magnitude of the charges present in the system.
- (ii) Distance separating the charges in the system.
- (iii) The medium separating the two electrically charged particles.
- (iv) Nature of the charges: positive or negative.
Limitations of Coulomb’s Law
Coulomb’s Law is derived under certain assumptions. Coulomb’s law is not applicable under the following conditions:
- If the charges are mobile. For the validity of Coulomb’s law, the charges must be stationary with respect to each other.
- If the electrical charges have an asymmetrical charge distribution. For the validity of Coulomb’s law charges must have a spherically symmetric distribution.
- If the charges overlap with each other. For the validity of Coulomb’s law, they must be distinct point charges.