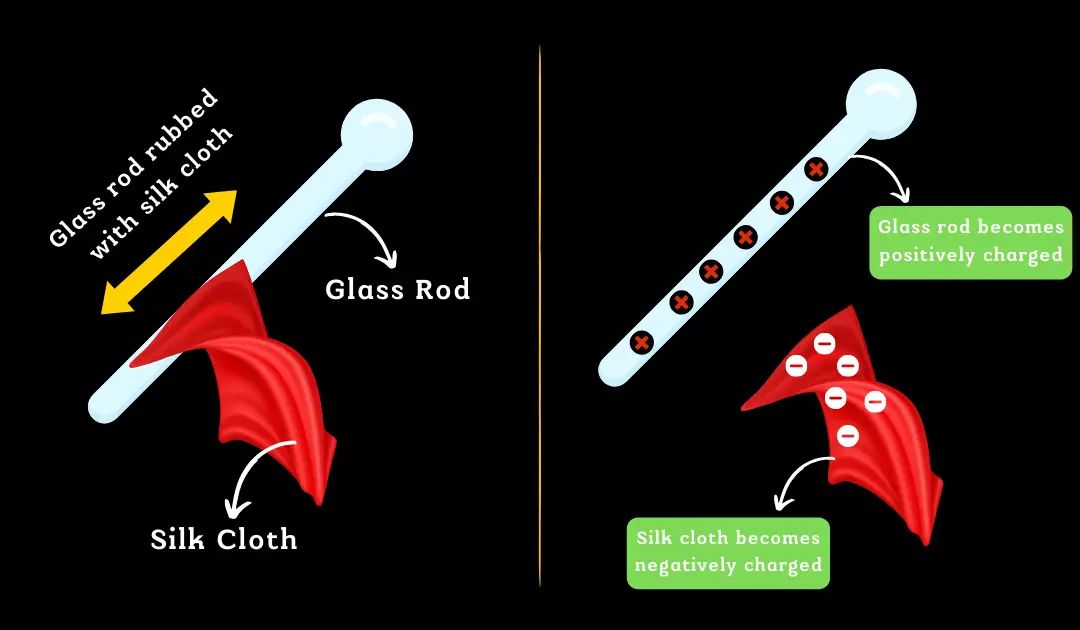
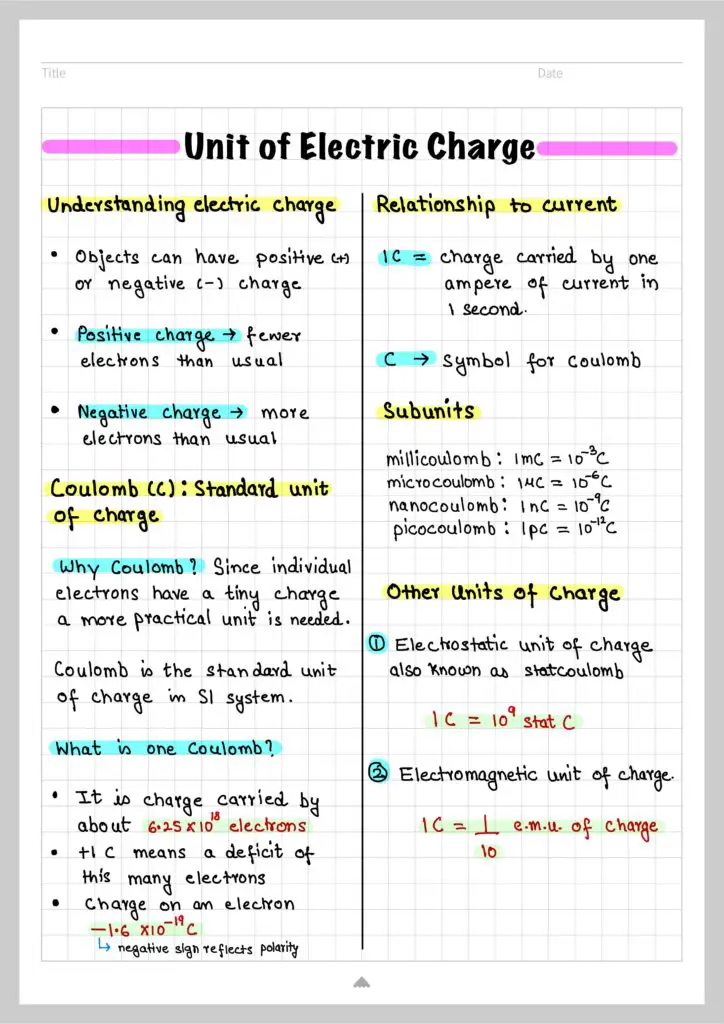
A body with a positive charge indicates it has fewer electrons than normal, while a negatively charged body has more electrons. Given the electron’s extremely small charge, using it as a standard unit of charge is impractical.
Due to the extremely small charge of an individual electron, using it as a base unit for measuring charge is impractical. Therefore, the Coulomb (C) is adopted as the standard unit of electric charge in the International System of Units (SI).
The Coulomb, as the unit of electric charge, helps in quantifying interactions in electrical circuits, the force between charged particles, and the structure of atoms.
The Coulomb (C)
Definition of the coulomb (C)
The magnitude of one Coulomb is equivalent to the charge carried by approximately $6.25 \times 10^{18}$ electrons. Mathematically, this is expressed as:
$$1 \, \text{Coulomb} = \text{Charge on} \, 6.25 \times 10^{18} \, \text{electrons}$$
Thus, when a body possesses a charge of +1 C, it signifies a deficit of $6.25 \times 10^{18}$ electrons compared to its neutral state. The charge of a single electron is quantified as $-1.6 \times 10^{-19}$ Coulombs, denoted by:
$$e = -1.6 \times 10^{-19} \, C$$
This negative value reflects the electron’s charge polarity. Determining the electron’s charge is a crucial experimental milestone in physics, supporting key principles in electrostatics and electromagnetism.
The Coulomb (C) is a fundamental unit of electric charge in the International System of Units (SI).
1 Coulomb is defined as the amount of charge transported by a constant current of one ampere in one second.
This unit is named after Charles-Augustin de Coulomb, an 18th-century French physicist known for his work on electrostatics.
Symbol and abbreviation for coulomb
The symbol for the coulomb is “C”. There is no separate abbreviation for the coulomb beyond its symbol.
Relationship with Ampere
The relationship between the coulomb and the ampere, which is the SI unit for electric current, is given by the equation:
\[ 1 \, \text{C} = 1 \, \text{A} \times 1 \, \text{s} \]
This equation means that one coulomb is equal to the amount of electric charge transferred by a current of one ampere flowing for one second.
Subunits of Coulomb
Because the Coulomb can represent a large quantity of charge, smaller units are often used in practical applications. These subunits are based on standard metric prefixes:
- Millicoulomb (mC): One millicoulomb is equal to one-thousandth of a Coulomb \((1 \, \text{mC} = 10^{-3} \, C)\).
- Microcoulomb (μC): One microcoulomb is equal to one-millionth of a Coulomb \((1 \, \mu C = 10^{-6} \, C)\).
- Nanocoulomb (nC): One nanocoulomb is equal to one-billionth of a Coulomb \((1 \, \text{nC} = 10^{-9} \, C)\).
- Picocoulomb (pC): One picocoulomb is equal to one-trillionth of a Coulomb \((1 \, \text{pC} = 10^{-12} \, C)\).
Other units of Electric Charge
In electrostatic CGS system, the unit of charge is known as electrostatic unit of charge (e.s.u of charge). It is also called statcoulomb (stat C)
$1C=3\times10^9 \, stat\, C$
In electromagnetic CGS system, the unit of charge is known as electromagnetic unit of charge (e.m.u of charge).
$1C=\frac{1}{10} \text{e.m.u of charge}$
Summary
Related Articles
Frequently Asked Questions
- What defines a positively or negatively charged body?
A positively charged body has fewer electrons than protons, resulting in a net positive charge. A negatively charged body has more electrons than protons, resulting in a net negative charge. - Why is the Coulomb used as the unit of charge?
The Coulomb is used due to the impracticality of using the charge of a single electron as a unit, given its extremely small magnitude. The Coulomb provides a more manageable scale for measuring electric charge. - How is the charge of an electron determined?
The charge of an electron is determined through experimental methods, such as the oil drop experiment conducted by Robert A. Millikan, which precisely measured the charge of an electron.