Electricity, recognized as a manifestation of energy, originates from diverse sources including chemical reactions, nuclear processes, thermal dynamics, and solar phenomena. Central to its nature is the movement and interaction of electric charges within various materials.
This article is about explaining nature of electricity. Before we proceed, let us first look into the concept of electricity and its existence.
The Concept of Electricity
We all are familiar with the importance of electricity in our day to day life. In addition to many other uses, it can be used for heating, lighting, and powering motors.
Electricity is the presence and flow of electric charge, primarily electrons, facilitated by electric fields. It is a versatile form of energy, harnessed from various natural sources, such as chemical reactions in batteries, the nuclear processes in the sun, or the flow of electrons in conductors.
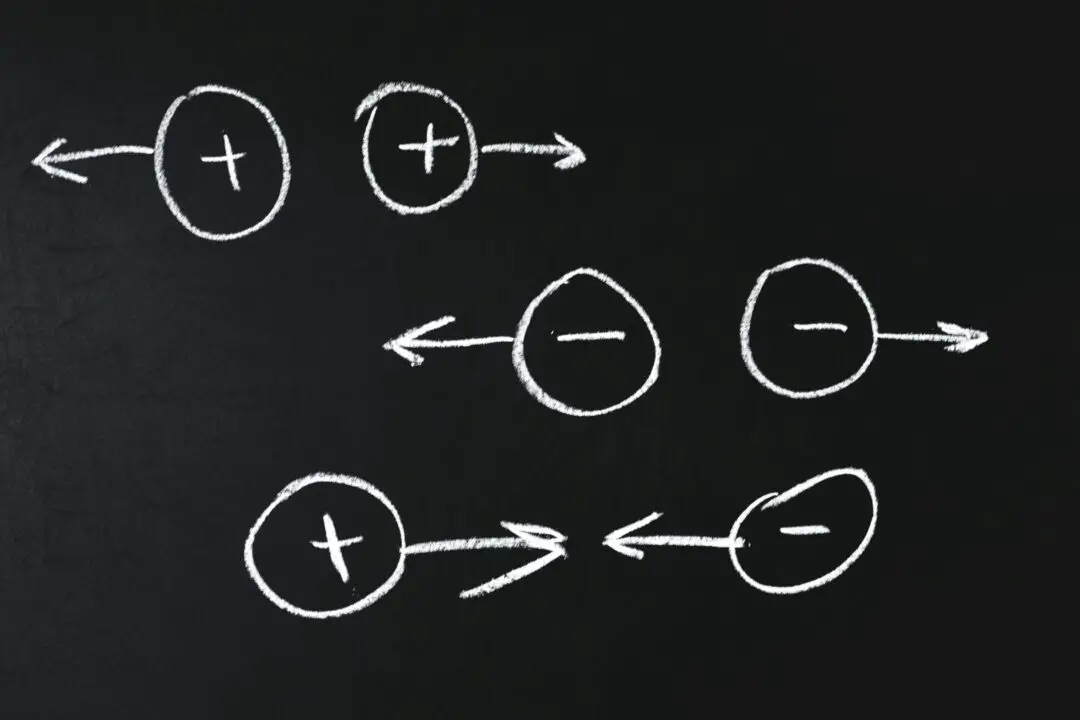
At its core, electricity is about the attraction and repulsion between charges. Positive and negative charges exert forces on each other, either pulling together or pushing apart. This interaction is the basis for electric currents – flows of charge which carry energy from one place to another.
The Structure of Matter
At the Macroscopic Level
Matter, in its broadest sense, encompasses all entities that occupy space and possess mass. This includes everything from the smallest particles to the largest structures in the universe.
The electrical properties of matter are intrinsically tied to its microscopic structure, particularly the behavior of electrons within atoms.
Zooming into the Microscopic Scale
At the microscopic level, matter reveals its intricate composition of molecules. Molecules are aggregates of atoms, linked by chemical bonds, which define the substance’s chemical properties. The variety in molecular architecture and composition accounts for the multitude of materials we encounter in our daily lives and scientific explorations.
Atoms: The Core of Molecules
Each molecule is composed of atoms, the fundamental units of chemical elements. Atoms are the building blocks that maintain their distinct identity during chemical interactions. Their size is remarkably small, typically in the order of a few angstroms (\(1 \, \text{angstrom} = 1 \times 10^{-10}\) meters). These atoms are not just physical entities but also possess electrical properties due to their constituent protons, neutrons, and electrons. The electrons, particularly those in the outermost shell, play a vital role in conductivity and electrical interactions.
Classification of Substances
Based on atomic makeup, substances are categorized into elements and compounds:
Elements: Pure substances consisting exclusively of one type of atom. For instance, a gold nugget is composed entirely of gold atoms.
Compounds: When atoms from different elements bond in fixed ratios, they form compounds. A classic example is water (H₂O), a compound of hydrogen and oxygen atoms in a 2:1 ratio.
Different elements and compounds have distinct electrical properties based on their atomic and molecular structures.
Electrical Nature at the Atomic Level
In physics, comprehending the electrical nature of atoms is essential. Each atom’s nucleus, located at its center, is composed of positively charged protons (\( p^+ \)) and neutral neutrons (\( n^0 \)). Encircling the nucleus, electrons (\( e^- \)) – negatively charged particles – occupy distinct energy levels.
This specific configuration of charged particles within an atom is fundamental to understanding the electrical properties of matter. Electrons, due to their negative charge and mobility, play a particularly crucial role. Their interactions within and between atoms significantly influence the electrical conductivity and the characteristic behavior of various materials. The way electrons move, share, or transfer between atoms underlies the conductive, insulating, or semiconductive nature of different substances.
This atomic-level electrical nature not only defines the inherent electrical characteristics of materials but also forms the basis for much of modern technology, from the simplest electrical circuits to the most complex electronic devices.
The Structure of an Atom
Nucleus: The Atom’s Core
At the center of an atom resides the nucleus, a compact and dense area that, despite occupying only a small fraction of the atom’s volume, contains the majority of its mass. The nucleus is composed of:
Protons (\( p^+ \)): These positively charged particles define the atomic number and, consequently, the identity of the element. The positive charge of protons plays a crucial role in the atom’s electrical properties.
Neutrons (\( n^0 \)): Neutrally charged particles that contribute to the nucleus’s mass without influencing its overall electric charge.
Electrons: The Atom’s Environs
Encircling the nucleus are electrons (\( e^- \)), each bearing a negative charge. These electrons are not randomly distributed; instead, they occupy specific energy levels or shells. Each shell can hold a certain number of electrons, and the arrangement of electrons within these shells is pivotal for the atom’s chemical properties and its interactions with other atoms.
Significance of Electron Shells: These shells are fundamental in understanding an atom’s reactivity, bonding capabilities, and electrical characteristics. The outermost shell, in particular, determines how an atom will interact with others, influencing the formation of molecules and various chemical reactions.
Electron Movement and Electric Current
Electrons are significant in both chemical bonding and electrical phenomena due to their mobility and charge. In the realm of electricity, it’s the movement of these electrons that predominantly drives electrical conductivity. When an external energy source is applied, electrons can be excited to higher energy levels or even move from one atom to another. This transfer or flow of electrons constitutes an electric current.
Electrical Characteristics of Atoms: The ability of atoms to gain, lose, or share electrons (often determined by the electron configuration in their outer shells) underlies the electrical nature of different materials. Conductors, insulators, and semiconductors exhibit their characteristic electrical behavior largely due to the electron configuration and mobility within their constituent atoms.
Modern Electron theory
Modern Electron Theory significantly expands our comprehension of atomic structure, integrating the concepts of electricity and electromagnetism. At its heart lies the role of electrons, which are negatively charged particles that orbit an atom’s nucleus. In a state of equilibrium, the quantity of electrons is equal to that of protons, maintaining the atom’s neutrality.
Crucially, it’s the behavior of these electrons, especially their movement and displacement, that underpins the generation of electrical phenomena. The transfer of electrons from one atom to another results in the production of an electric current, a fundamental aspect in understanding both electrical conductivity and the response of materials to electric fields.
The evolution of this theory is credited to the groundbreaking work of luminaries such as J.J. Thomson, Robert A. Millikan, Sir Ernest Rutherford, and Niels Bohr. Their collective contributions laid the groundwork for our current understanding of electricity and its atomic foundations, shaping our modern perspective on how electrical processes operate at the atomic level.
Electrical Nature of Matter
Matter inherently exhibits electrical properties due to its atomic structure, which comprises electrically charged particles: protons and electrons. The electrical state—whether charged or neutral—of any material is dictated by the balance of these particles.
Electrical Neutrality: An object is electrically neutral when its number of protons (positively charged) matches its electrons (negatively charged). For example, a piece of paper typically demonstrates this balance, maintaining an equal count of protons and electrons, thus having no net electrical charge.
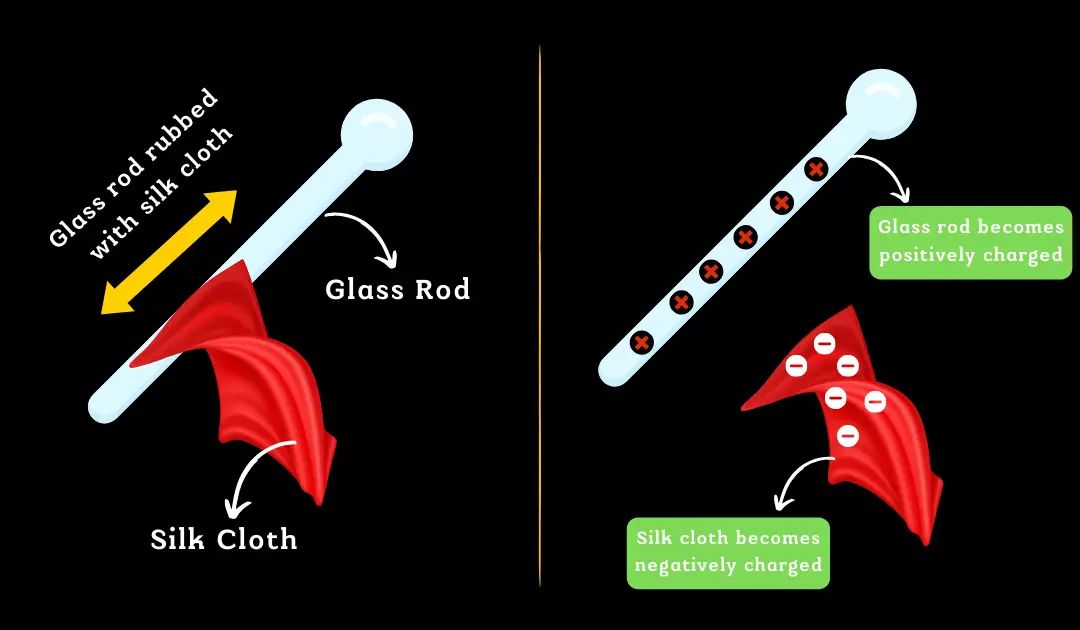
Positive Charge Formation: An object assumes a positive charge when it loses electrons. This deficit leads to a predominance of protons, resulting in a net positive charge. Essentially, a positively charged material has fewer electrons compared to its neutral state.
Negative Charge Formation: Conversely, an object becomes negatively charged upon gaining extra electrons, surpassing the number of protons. This surplus of electrons results in a net negative charge, meaning a negatively charged object contains more electrons than when it is neutral.
A key point to note is the mobility of electrons; they can be readily displaced or shared, unlike protons which are firmly anchored within the nucleus. This characteristic of electrons is central to the formation of both positive and negative charges in materials.