Let us try to understand first what a dipole is? More specifically, what an electric dipole is? Then we will sum up the electric dipole concept with few examples.
What do we mean by the terms electric dipole and electric dipole moment?
The word “dipole” literally means two poles. In electromagnetism, there are two kinds of dipole:
- electric dipoles and
- magnetic dipoles.
Electric Dipole
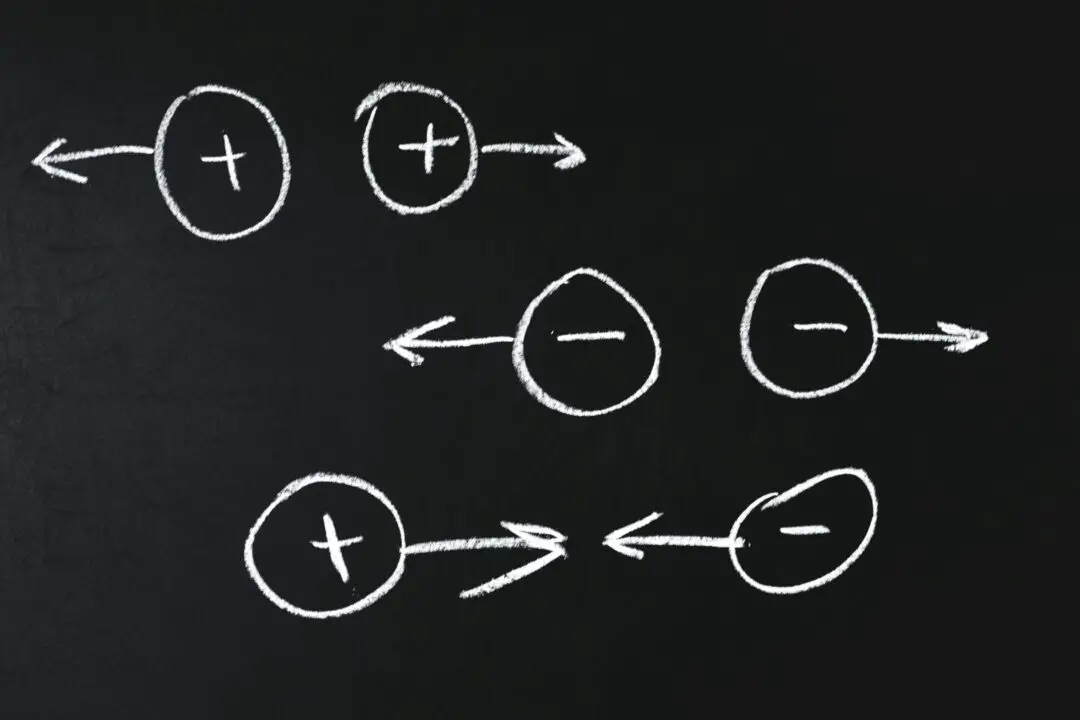
An electric dipole is defined as a pair of point charges equal in magnitude but opposite in charge $(+Q , -Q)$, separated by a distance $(2a)$.
Magnetic Dipole
A magnetic dipole is the limit of either a closed loop of electric current or a pair of poles as the size of the source is reduced to zero while keeping the magnetic moment constant.
Electric Dipole moment
Electric dipole moment is the charge possessed multiplied by the distance separating them. It is the strength associated with an electric dipole.
$p=q\times 2a$
where,
- where $p$ is the electric dipole moment pointing from the negative charge to the positive charge.
- $q$ is the magnitude of charge.
- $2a$ is the separation between two charges.
The SI units for electric dipole moment are Coulomb-meter $(C⋅m)$; however, a commonly used unit in atomic physics and chemistry is the debye $(D)$ where 1 debye is $3.34\times10^{−30} Cm$.
The dipole moment is a vector quantity that has both magnitude and direction. In physics as a convention, the direction of electric dipole moment is taken along the line from negative charge toward positive charge. While in Chemistry, this convention is the opposite, that is, from positive to negative. The line along the direction of an electric dipole is called the axis of the dipole.
Significance of Electric Dipole – Polar vs Non-polar molecules.
Molecules are composed of atoms of the same or different elements. Every element has its own characteristic properties with which it either attracts or repels an electron.
Polar Molecules
In Polar molecules, the electron density is unequally shared between the atoms i.e. centers of negative charges and positive charges do not coincide. Therefore, they have a permanent electric dipole moment, even in the absence of an electric field. These molecules are called polar molecules.
The water molecule, $H_2O$, is an example of polar molecules. Various materials give rise to interesting properties and important applications in the presence or absence of electric fields
Non-Polar Molecules
In non-polar molecules, the electron density is equally shared between the atmos i.e. the centers of positive charges and negative charges lie at the same place. Therefore, their dipole moment is zero. $CO_2$ and $CH_4$ are of this type of molecule. These molecules are called non-polar molecules. However, they can develop a dipole moment when an electric field is applied.
Types of electric dipole for molecules?
For molecules, there are three types of dipoles: –
- Permanent dipoles
When constituent atoms in a molecule have a substantially different electronegativity; then electron density is unequally shared between atoms. Polar molecules are examples of permanent dipoles. E.g., $HCl$, $CHCl_3$ - Instantaneous dipoles
The electron distribution around an atom undergoes fluctuations in time resulting in creation of instantaneous electric fields which results in transient spatial electron redistribution in the neighbouring atoms and molecules, creating instantaneous dipoles. - Induced dipoles
Due to an external force, a dipole can be produced known as an induced dipole.
Molecules which are examples of the common electrical dipole?
-
Potassium Bromide $(KBr)$
- Potassium Bromide has one of the highest dipole moments (10.41 D) because it is an ionic compound. This happens because of the significant difference in electronegativity between potassium and bromine
- Bromine is a highly electronegative element which results in electron density being more concentrated on it. Therefore, In an Potassium Bromide molecule, Bromine atom has a partial negative charge and Potassium atom has a partial positive charge.
- Potassium Bromide is an example of permanent dipole.
-
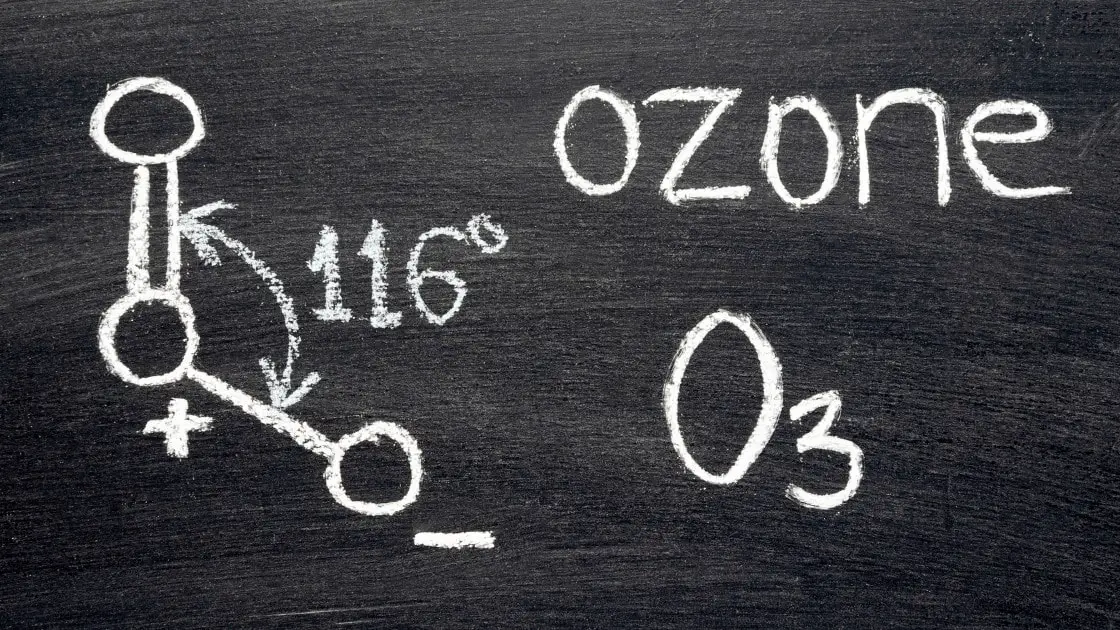
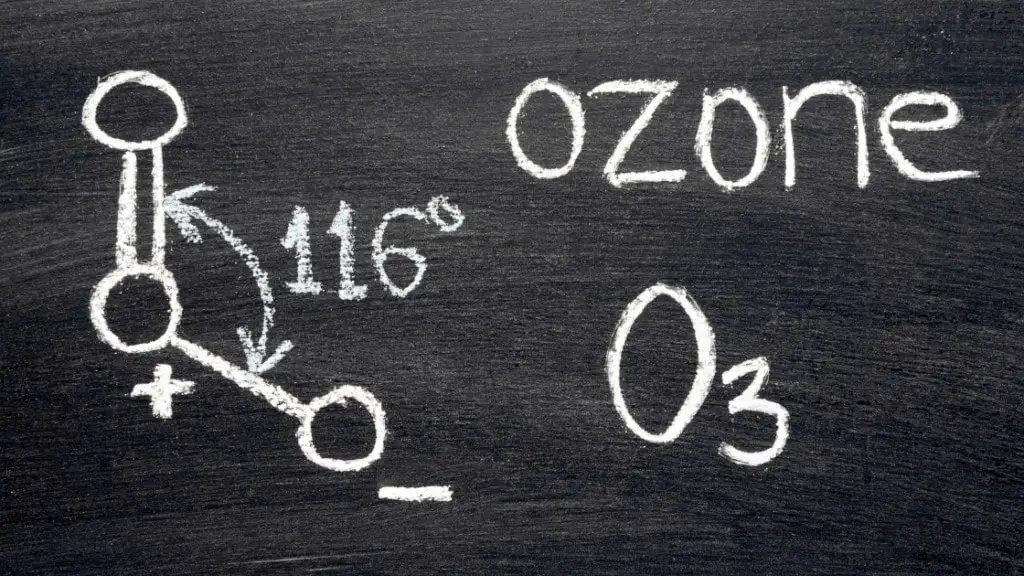
Ozone $(O_3)$
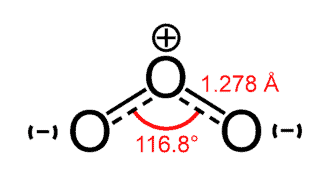
- Ozone is a polar molecule with a dipole moment of $0.53 D$.
- A Ozone molecule is composed of three Oxygen atoms.
- It has a bent molecular structure.
- The $O – O$ bond distances are $127.2 pm$
- The $O – O – O$ bond angle is 116.78°.
- Thus, due to the unequal electron density distribution between Oxygen atmos, Ozone molecule is an example of electric dipole.